Abstract
Myelodysplastic syndrome (MDS) is a hematological clonal stem cell disease. Recurrent splicing factors mutations are reported in 50% of MDS. Interestingly, mutations in the splicing factor gene SF3B1 are over-represented in MDS with ring sideroblasts (MDS-RS), co-occurring in up to 90% of patients. In MDS-RS, anemia is the major clinical manifestation. Erythropoiesis stimulating agents (ESAs) are used to treat anemia; however, the overall response rates are 20% to 40% with a duration of response of 18-24 months. New therapeutic options are needed to improve response to ESAs treatment and delay red blood cell transfusion, which are associated with acute myeloid leukemia progression and increase in morbidity.
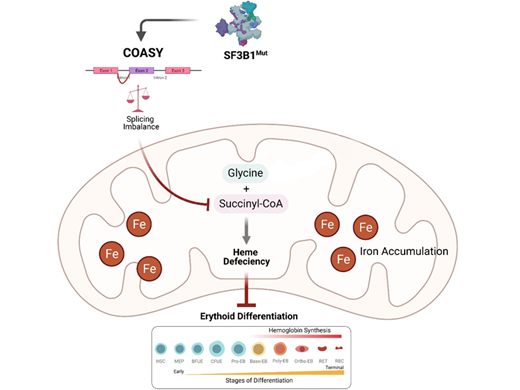
Mutations in SF3B1 modify the recognition pattern of the 3' splice site and lead to subsequent mis-splicing of its targets. To identify critical mis-splicing events involved in the erythroid differentiation blockage, we performed splicing analysis on RNA sequencing generated from hematopoietic stem/progenitor cells undergoing differentiation. Three MDS primary samples harboring SF3B1 mutations and three age-matched healthy donors cultured under normoxia and hypoxia conditions were initially used for the analysis. High depth RNA sequencing and differential splicing analyses using rMATS identified 2,845 mis-spliced events including 200 shared between hypoxia and normoxia conditions. Here, using a cohort of 42 MDS samples, we report the mis-splicing of the coenzyme A synthase (COASY) transcript. Heme synthesis relies on succinyl-CoA synthesis, and its production itself depends on the availability of cellular CoA. We thus hypothesised that COASY mis-splicing is a key driver of ineffective erythropoiesis in MDS-RS patients.
In primary hematopoietic cells, COASY is upregulated during erythroid differentiation and its silencing in CD34 + cells severely impedes the generation of mature erythroid cells CD71 - CD235a + and causes disruption in heme production. Functional characterisations of the CRISPR-CAS9 edited K562 SF3B1K700E and the SF3B1-mutated HNT-34 cell lines confirmed that COASY mis-splicing impairs COASY protein synthesis that ultimately results in 60% loss of the protein. Metabolomic analysis showed that COASY mis-splicing depletes cells in CoA and succinyl-CoA metabolites, however this phenotype can be rescued by supplementation with vitamin B5, a CoA precursor. Consequently, we showed in vitro that saturating the 40% of remaining COASY enzyme with vitamin B5 or supplementing medium with its downstream by-product, succinyl-CoA, improved erythropoietic differentiation in MDS SF3B1mut patients.
In summary, our results for the first time show that SF3B1 mutations induce coenzyme A synthase (COASY) transcript mis-splicing, that consequently leads to measurable defects in metabolites essential for heme biosynthesis. Our report reveals a novel critical role of COASY in regulating normal bone marrow erythropoiesis through control of succinyl-coA during human erythroid differentiation. Remarkably, partial loss of the coenzyme A synthase in MDS-RS patients leads to disruption in the erythroid lineage as well as heme deficiency, that can be rescued by exogenous treatment with vitamin B5 or succinyl-CoA. Therefore, vitamin B5 could represent a very attractive agent to combine with existing treatments in order to increase erythroid maturation and delay red blood cell transfusion dependency in MDS-RS patients.
Graphical representation: SF3B1 mutant causes mis-splicing in COASY that results in loss of protein. Deficiency in COASY triggers a downregulation of succinyl-CoA that is involved in the rate limiting step of heme synthesis. Heme deficiency subsequently impairs erythroid differentiation. Treatment of MDS SF3B1 mutant cells with vitamin B5 (precursor of CoA), or succinyl-CoA, rescues erythroid differentiation.
Platzbecker: Geron: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Celgene/BMS: Honoraria; Novartis: Honoraria; AbbVie: Honoraria. Wiseman: Bristol Myers Squibb: Consultancy; Novartis: Consultancy; StemLine: Consultancy; Takeda: Consultancy; Astex: Research Funding. Gribben: Abbvie: Honoraria; AZ: Honoraria, Research Funding; BMS: Honoraria; Gilead/Kite: Honoraria; Janssen: Honoraria, Research Funding; Morphosys: Honoraria; Novartis: Honoraria; Takeda: Honoraria; TG Therapeutis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal